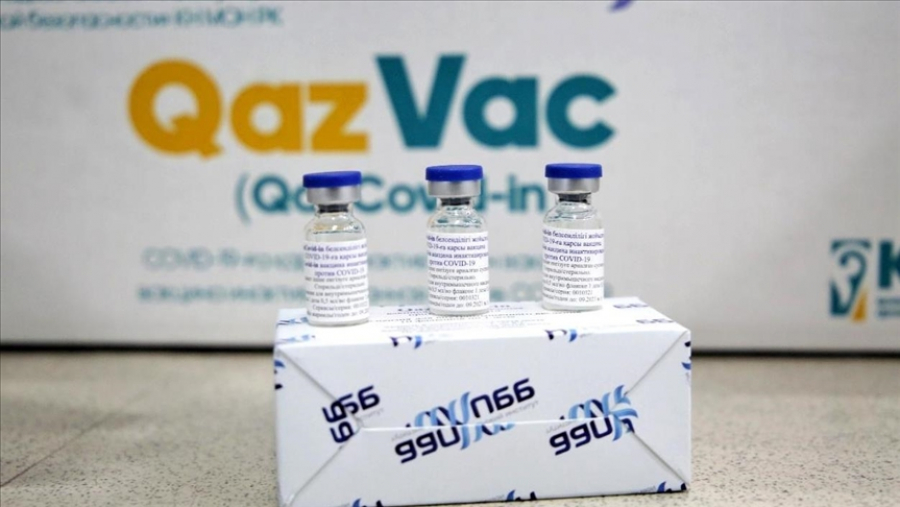
Another Kazakh domestic vaccine against coronavirus is undergoing clinical trials. Scientists at the Research Institute for Biological Safety Problems worked for a year on the development of the vaccine called QazCoVac-P. The vaccine will be tested in two phases. The current stage of the trial involves volunteers between 18 and 50 years of age. They will have to undergo a thorough medical examination before vaccination. A total of 244 people will take part in the trials, 44 in the first phase and 200 in the second.
“We will vaccinate 44 people in the first phase. We are now in the process of recruiting clinical study volunteers. First, we are going to get everyone screened for various diseases, allergies, and so on. In addition, all coronavirus vaccines can have side effects such as fever and weakness,” said Nurlan Abildayev, Chief Doctor, General Hospital, Taraz city.
“22 volunteers will be injected with the vaccine, and another 22 with placebo. The safety of the vaccine will be studied first, as well as immunogenicity. The second phase, which will include 200 volunteers, will also be focusing on the vaccine immunogenicity and safety. Placebo is used for comparison purposes. We use sodium chloride. We need to know how exactly the vaccine affects the human body compared to, figuratively speaking, a fake medicine,” said Berik Khairullin, Chief Researcher, Research Institute for Biological Safety Problems.
The vaccine has already been tested on animals. However, it is more difficult to study the drug in humans as everyone has a different immunological state. Age and place of residence also make a difference, experts say. In case of successful clinical trials, doses of the QazCoVac-P will be administered at 21-day intervals. Scientists also reported that the Institute is working on three other vaccines against coronavirus.
Translation by Assem Zhanmukhanova
Editing by Saule Mukhamejanova