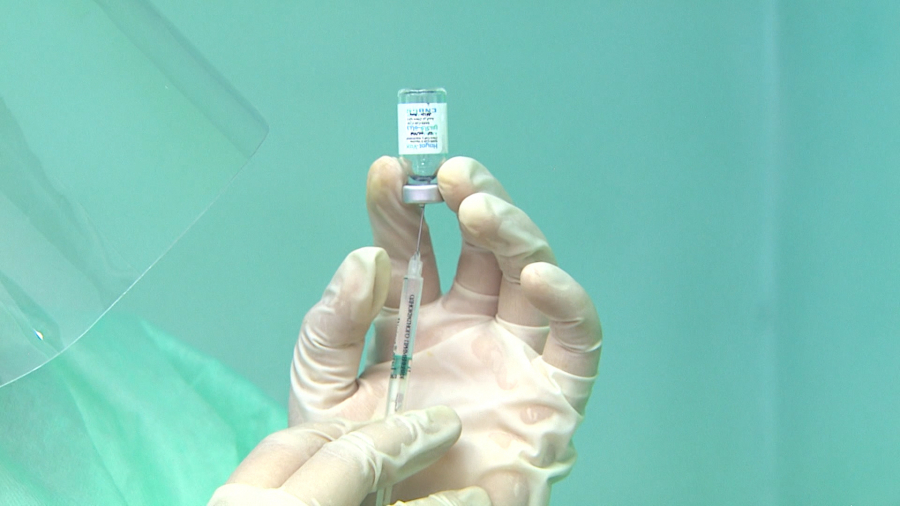
Chinese-made vaccine CoronaVac has obtained a temporary registration in Kazakhstan. Licensing procedures are accelerated in the pandemic conditions. The medicine is approved by the World Health Organization, is included in the list of vaccines suitable for use, and meets international safety and efficacy standards. In general, CoronaVac was approved in 25 countries. Over 300 million people have already received this vaccine. Kazakh Health Ministry is supposed to approve all accompanying documents for the medicine in the next few days. Instructions for the use of the vaccine will be worked out on their basis. After that, the medicine will be available at all vaccination points of the country. This is the fourth vaccine that was included in the list of those already used in the country.
“Its properties are well studied, its effectiveness is published and it’s about 80 percent. There are temporary side effects when using Sinovac, at the level of 30 to 40 percent, they are disappear without a trace. That is, the vaccine is safe,” said Alexander Shustov, Head of Laboratory for Genetic Engineering at the National Center for Biotechnology.
Translation by Saniya Sakenova
Editing by Galiya Khassenkhanova